
Medical device alerts hit four-year high
The number of alerts issued by the Medicine and Healthcare products Regulatory Agency (MHRA) has hit a four-year high of 35 in the past year*, up from 32 last year and more than double the 17 alerts issued three years ago, says City-headquartered law firm RPC.
- Changing regulatory environment drives need for product recall insurance
- Medical device alerts double in three years
RPC says the figures suggest that the need for medical device manufacturers to take out specific product recall insurance is rising, with full recalls increasing as a proportion of all medical product alerts. A number of alerts issued by the MHRA in 2018/19 involved a full product recall or an instruction to destroy remaining products.
RPC says that businesses that recall medical devices can face a wide range of costs, including the costs of shipping, storage and disposal, refunding purchasers, and increased staffing costs. Insurers have in recent years begun to offer tailored Product Recall & Contamination policies to cover these costs.
These specialist policies are increasingly required in addition to Product Liability policies, which only cover losses as a result of claims by parties who suffer injury or damage due to a defective product.
Peter Rudd-Clarke, Legal Director at RPC, comments: “Product Recall insurance should be considered as a key part of medical device manufacturers’ risk management.”
The firm adds that the Medical Devices Regulation 2017 that will fully apply in 2020 will toughen the requirements on medical device manufacturers to self-report when problems with devices are discovered.
The latest information from the MHRA suggests that medical device manufacturers are taking a low-risk approach, and are increasingly engaging with the MHRA where issues may exist with a product. This suggests that the public is benefitting from a tighter regulatory regime designed to improve the safety levels of products placed on the market.
These increased reporting requirements are also likely to drive sales of specialist product recall insurance policies.
Almost three-quarters of alerts concern implanted devices
RPC says that almost three-quarters of alerts issued by the MHRA in the past year involved the two most critical classes of devices** – those implanted into the body, such as pacemakers, replacement heart valves and breast implants.
Alerts issued by the MHRA in the past year concerned a range of devices, including:
- A heart monitor which risks battery packs exploding due to overheating
- A blood and IV fluid warming device, which could leach unsafe levels of aluminium into the bloodstream of users
- Knee joint replacements which risk early failure due to loosening parts
Adds Peter Rudd-Clarke: “The need for specialist product recall policies is only going to increase for medical device manufacturers over the coming years. The 2017 Regulation takes effect next year which may lead to recalls at an earlier stage of the product lifecycle if there are questions over a product's performance.”
“A product recall can represent a huge cost to a medical device manufacturer, and much of it is not covered by a standard product liability policy.”
Four-year high in warnings issued over faulty medical devices
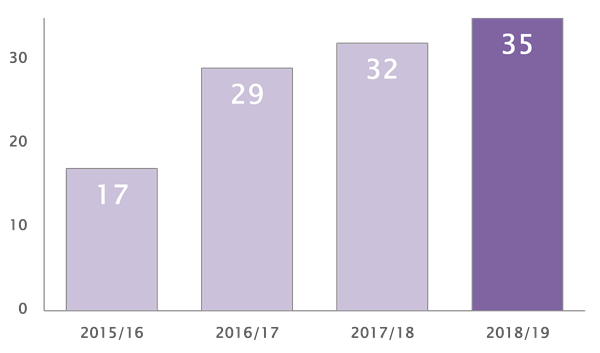
* Year-end June 30 2019. Source: MHRA
** Medical Device Directive Class IIb and Class III devices
